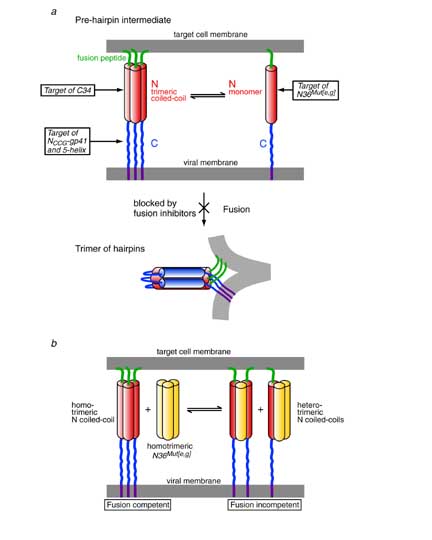
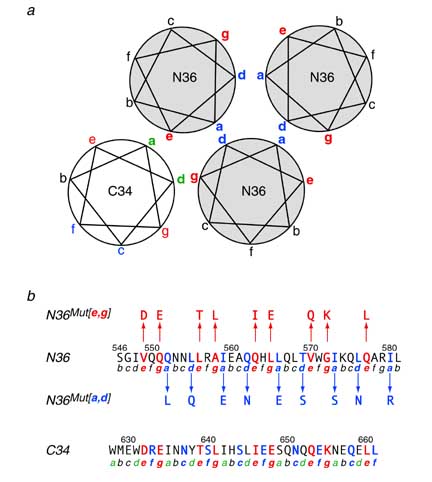
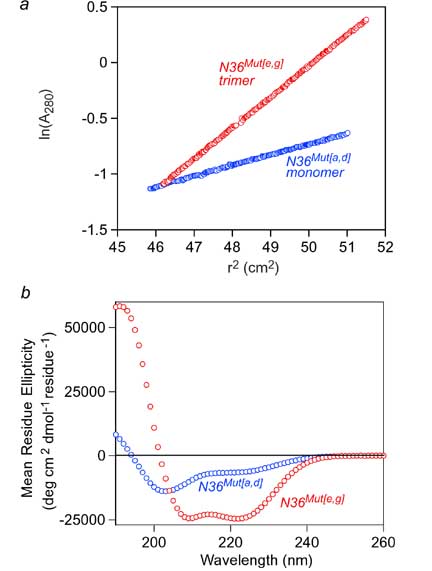
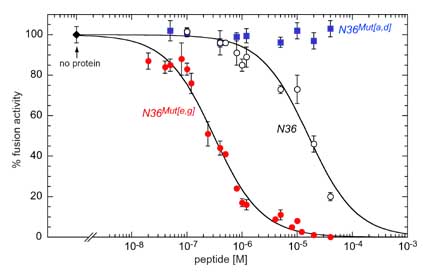
N36Mut[e,g] inhibits HIV-1 Env-mediated cell fusion 50-fold more effectively than the native N36 peptide. Since the residues that interact with the C-helix have been mutated in N36Mut[e,g], and since N36Mut[e,g] does not interact at all with the C34 peptide derived from the C-helix, N36Mut[e,g] must act by forming heterotrimers with the internal trimeric coiled-coil of N-helices in the pre-hairpin intermediate state of gp41.
Bewley, C.A., Louis, J.M., Ghirlando, R. & Clore, G.M. (2002) Design of a novel peptide inhibitor of HIV fusion that disrupts the internal trimeric coiled-coil of gp41. J. Biol. Chem. 277, 14238-14245. pubmed PDF