The Oct and Sox transcription factors control mahy different aspects of neural development and embyogenesis, often binding to adjacent sites on the DNA, and interacting with one another through their DNA binding domains to regulate transcription synergistically. Oct proteins contain two DNA binding domains (POUS and POUHD) connected by a flexible linker, which interact with DNA in a bipartite manner.
Residual dipolar coupling measurements on the binary Oct1·DNA complex reveal that the two domains are characterized by distinct alignment tensors (with different rhombicities) in a negatively charged liquid crystalline medium of phage pf1. This difference is due to a microscopic dissociation/association process for the weaker POUS domain in the binary complex. The populations of the hemi-associated states with POUS or POUHD unbound to DNA are estimated to be ~1% and 0.005%, respectively.
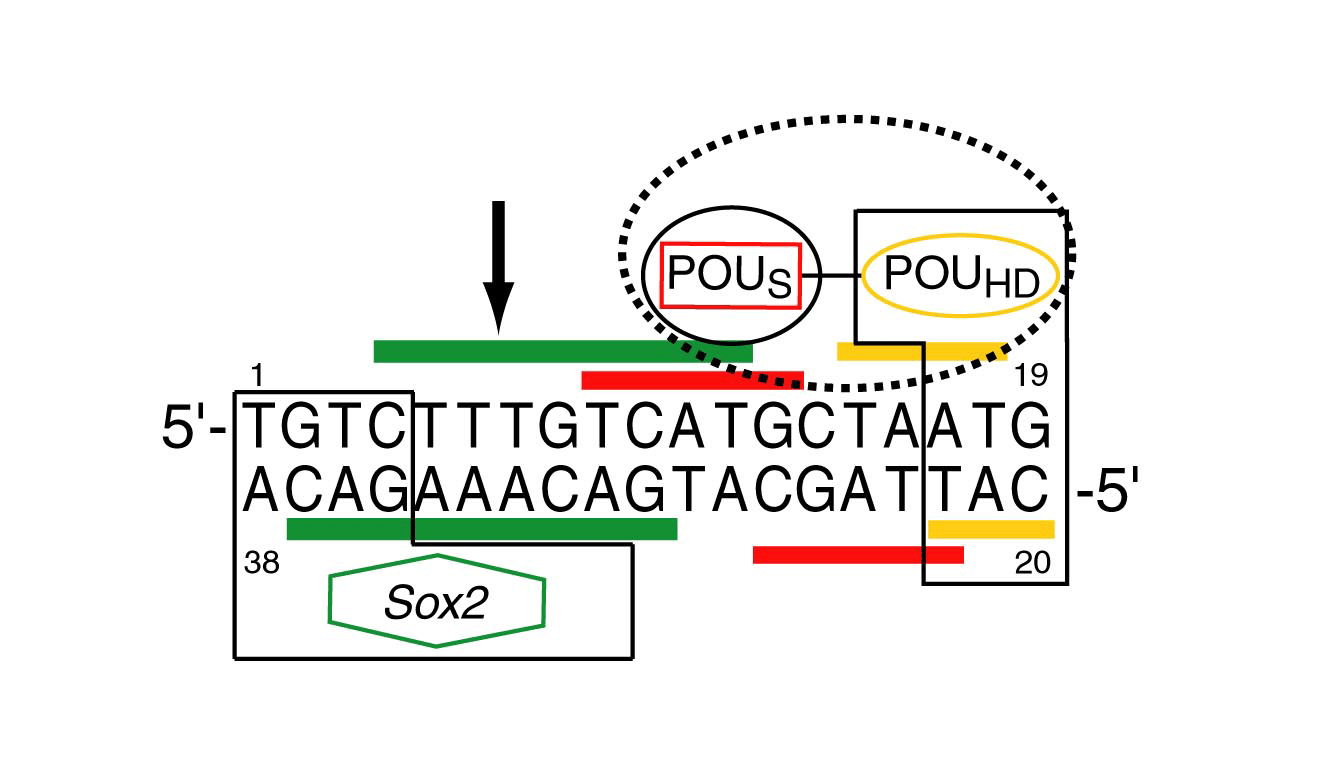
Upon binding of Sox2 to an adjacent site in the Hoxb1 regulatory elemet, all components of the ternary Oct1·Sox2·Hoxb1-DNA complex share a single alignment tensor in phage pf1. This indicates that ternary complex formation must increase the affinity of Oct1 for DNA by a minimum of 10-20 fold.
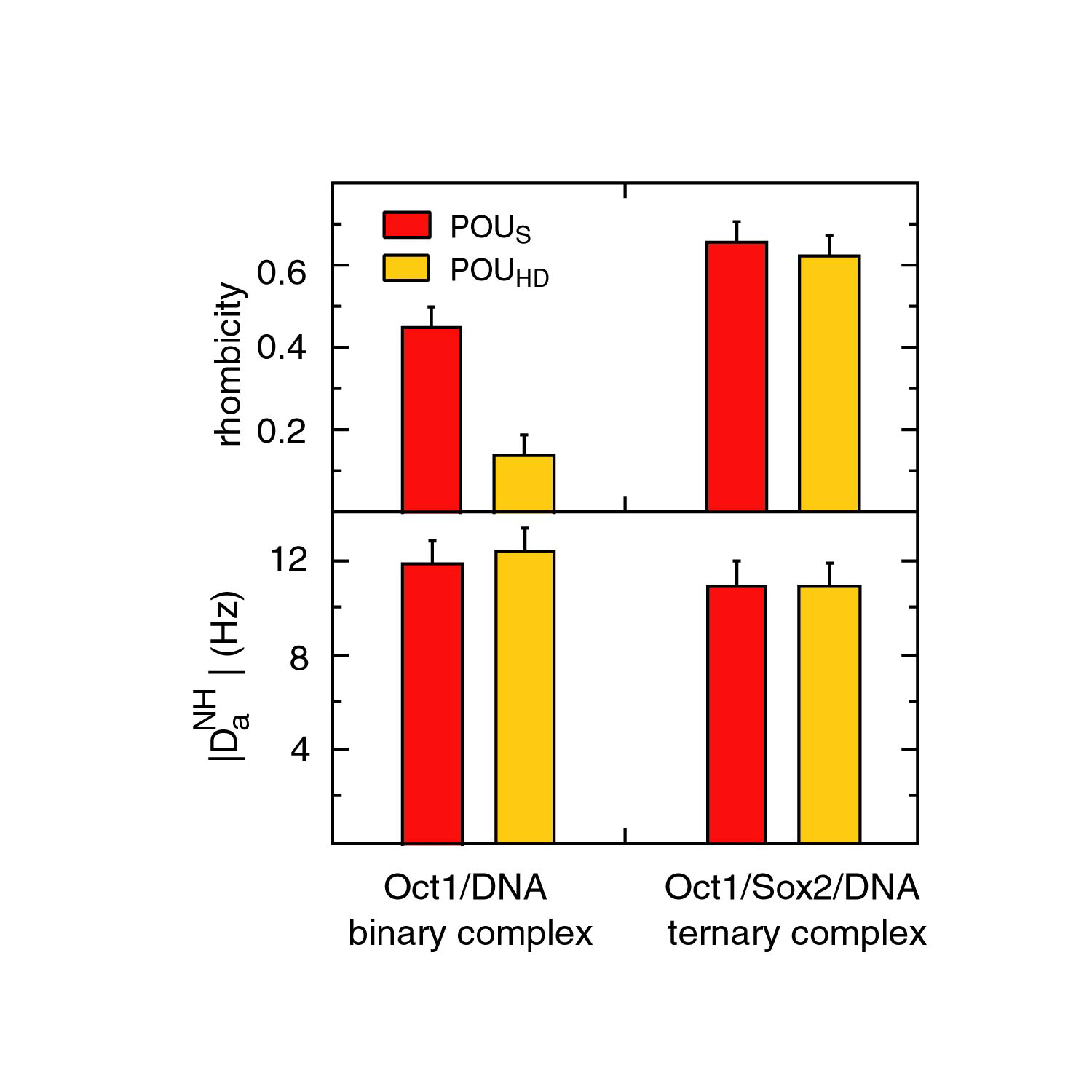
In the ternary complex, POUS and POUHD, as well as SOX2, have the same alignment tensor (i.e. same Da and rhombicity) indicating that they tumble as a single rigid body. In the binary Oct1·DNA complex, however, the alignment tensors for POUS and POUHD are different. Since the dipolar couplings for the binaary Oct1·DNA complex recorded in phage pf1 and PEG/hexanol are highly correlated, and since PEG/hexanol does not interact with free Oct1, these results indicate that POUS and POUHD adopt more than one orientation relative to one another in the binary complex. Since the affinity of POUHD for DNA is two orders of magnitude larger than that of POUS, this suggests that POUS binds to DNA in two or more orientations in the binary complex.
The solution NMR structure of the 42 kDa Oct1·Sox2.·Hoxb1-DNA complex was determined by novel procedures based on orientational restraints from dipolar couplings and conjoined rigid body/torsion angle dynamics.
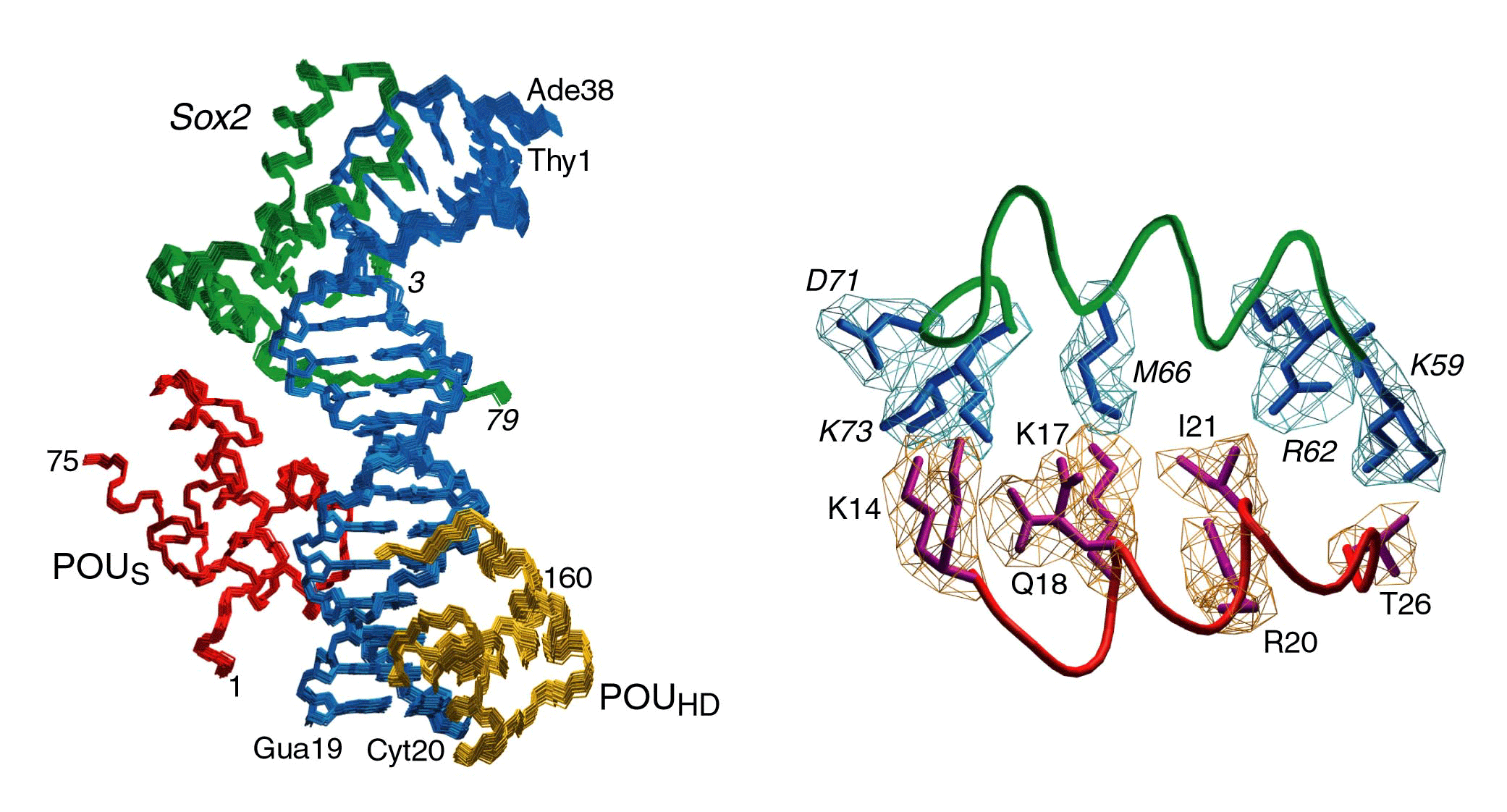
The structure of the ternary complex reveals that Sox2 and POUS interact through a predominantly hydrophobic interface surrounded by a ring of electrostatic interactions.
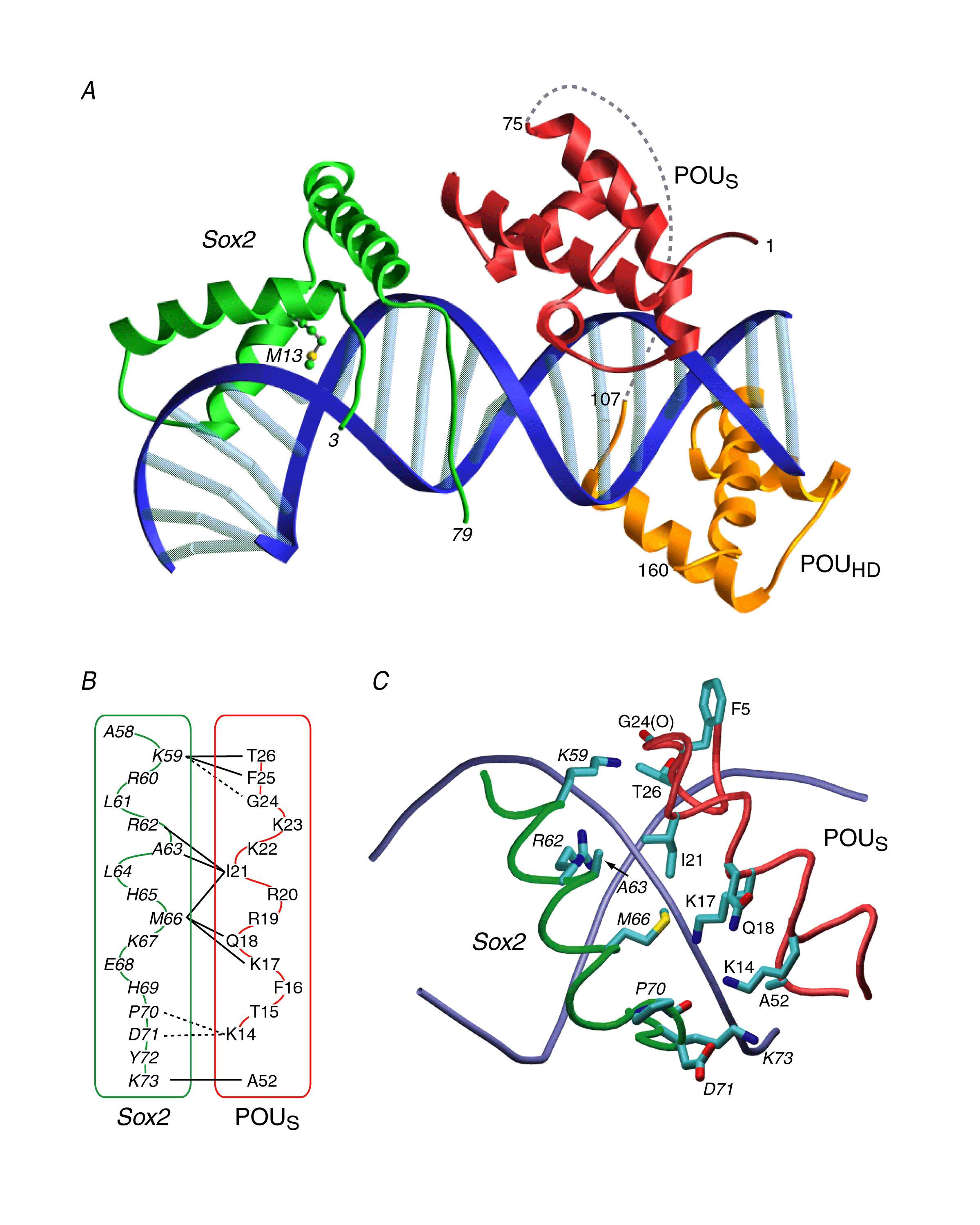
These observations suggest a mechanism of combinatorial control involving direct protein-protein interactions on the DNA whereby Oct1 in conjunction with a co-interacting transcription factor provide cell specific transcription regulation.
361. Williams, D.C., Cai, M. & Clore, G.M. (2004) Molecular basis for synergistic activation by Oct1 and Sox2 revealed from the solution structure of the 42 kDa Oct1·Sox2·Hoxb1-DNA ternary transcription factor complex. J. Biol. Chem. 279, 1449-1457. Pubmed PDF